|
FLUORINE IN GRANITE FLUIDS BY EXPERIMENTAL ESTIMATIONS Institute of Experimental Mineralogy RAS, Chernogolovka, Moscow region, Russia, aksyuk@iem.ac.ru
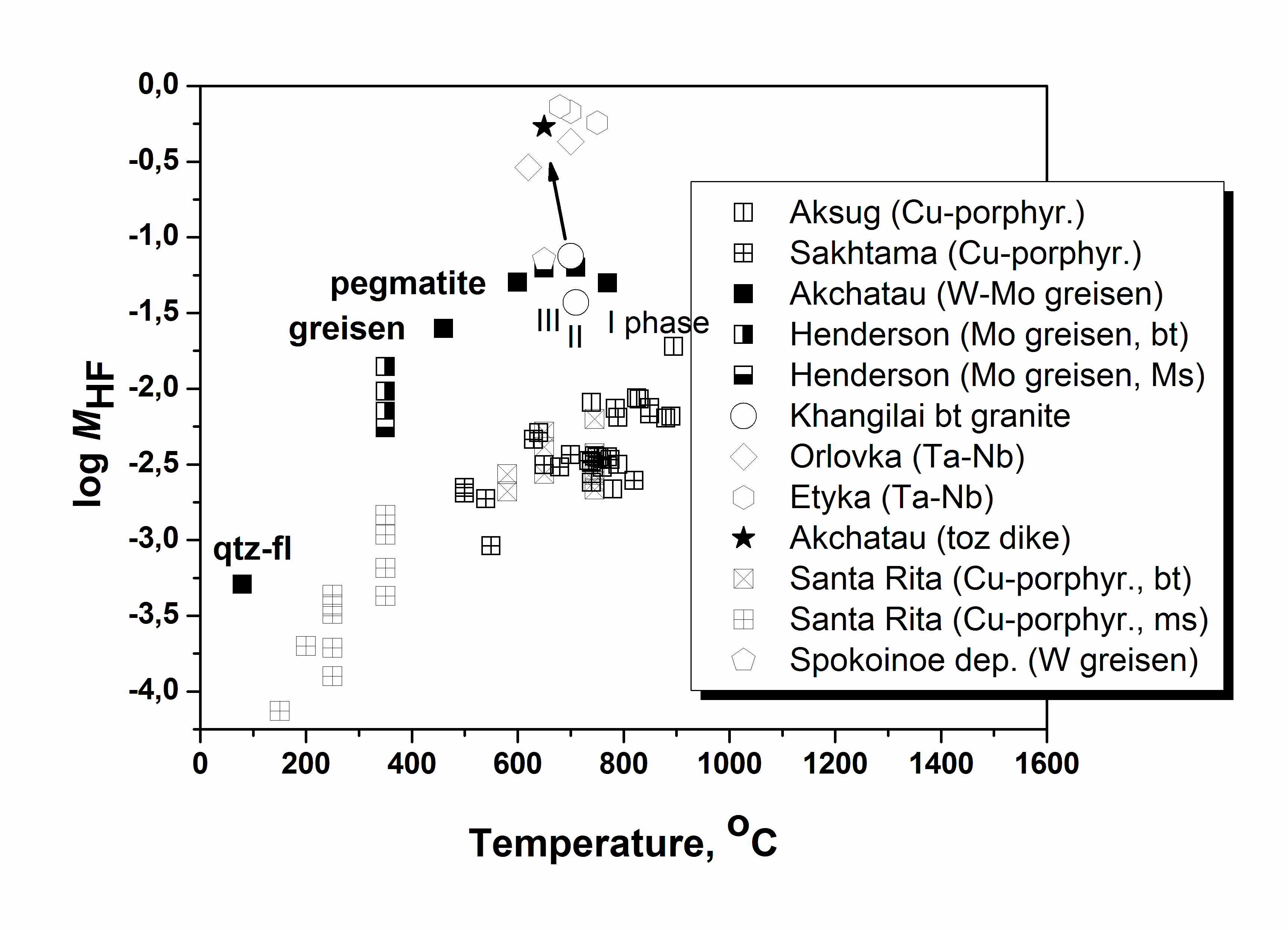
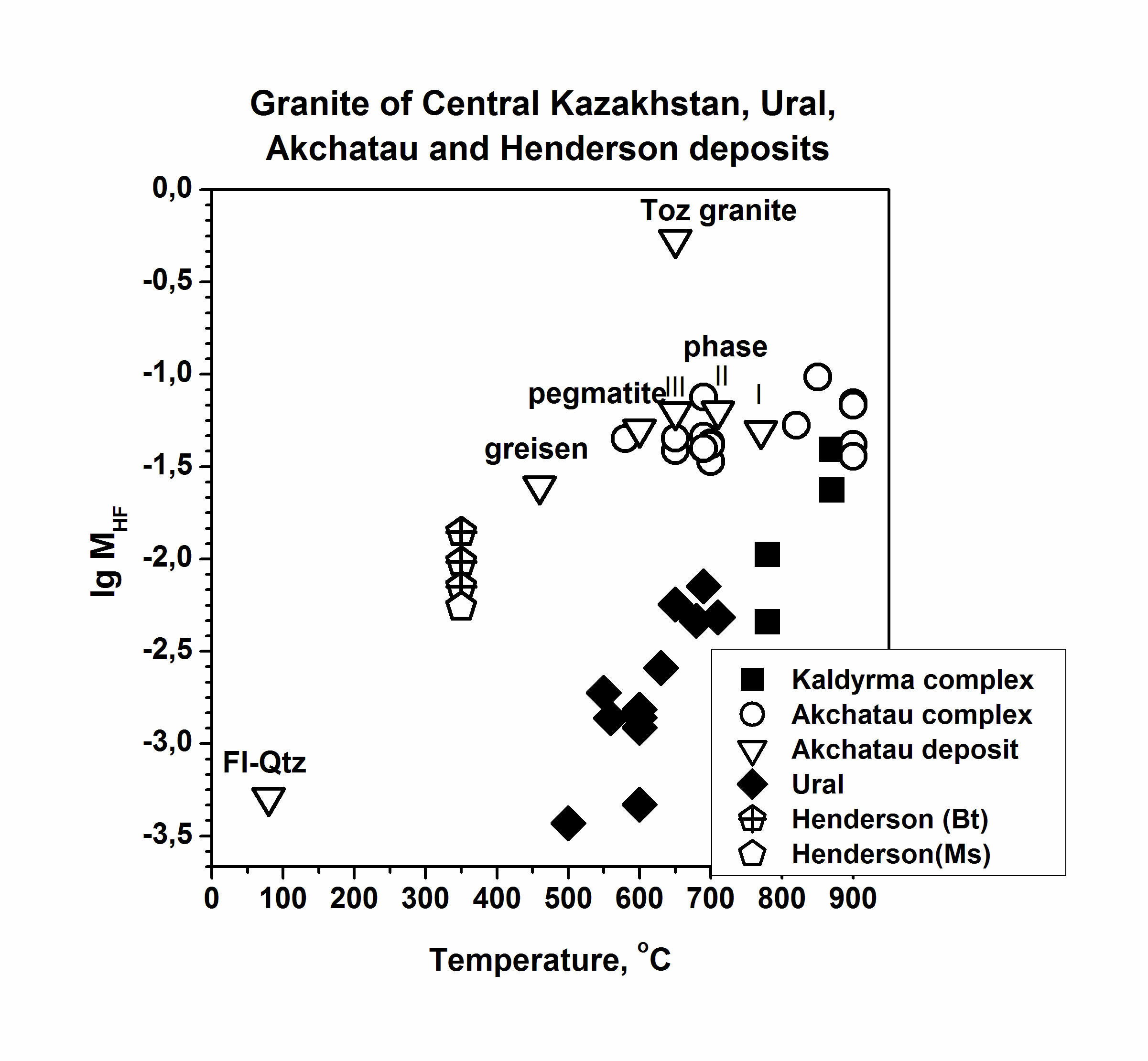
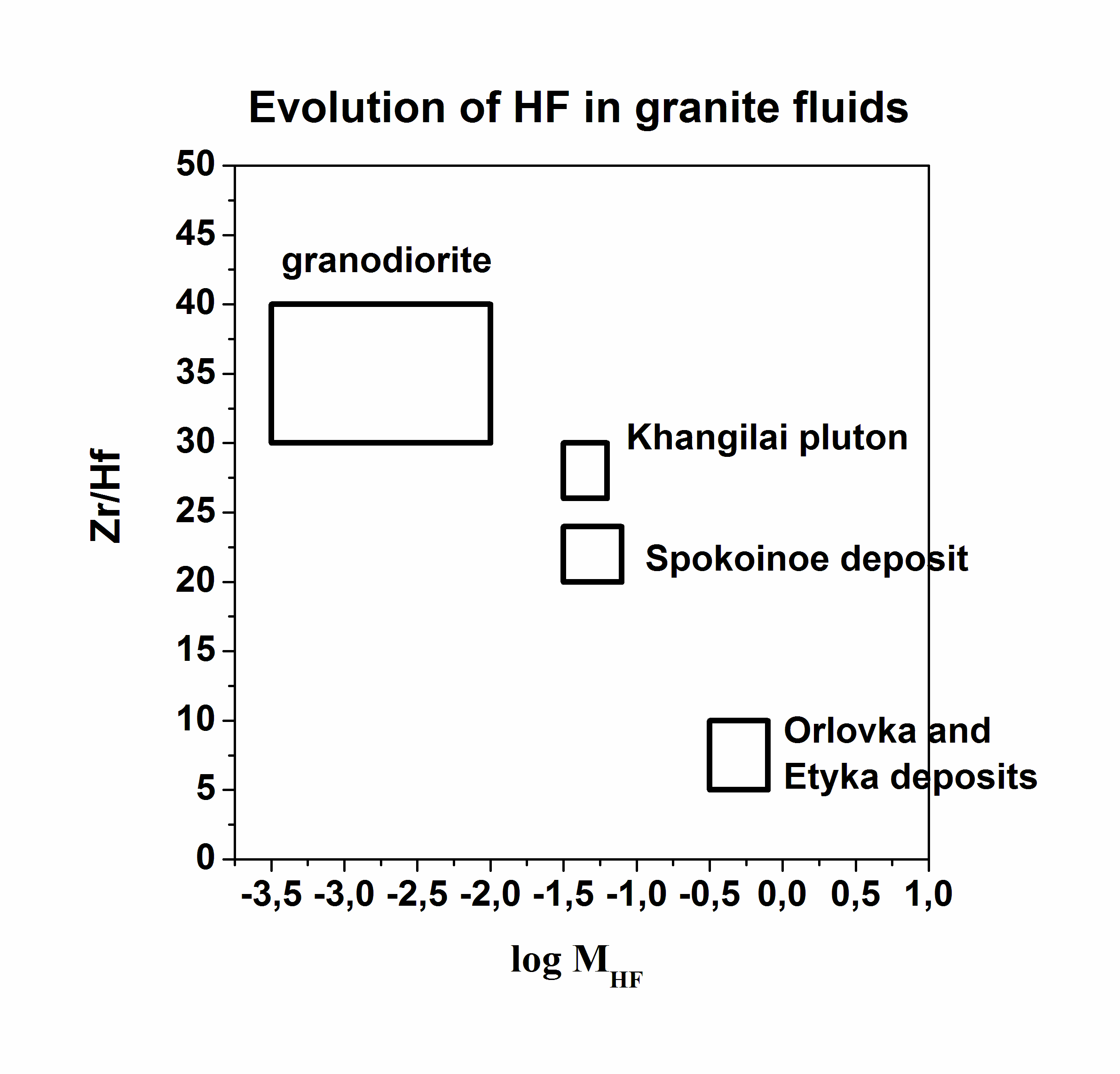
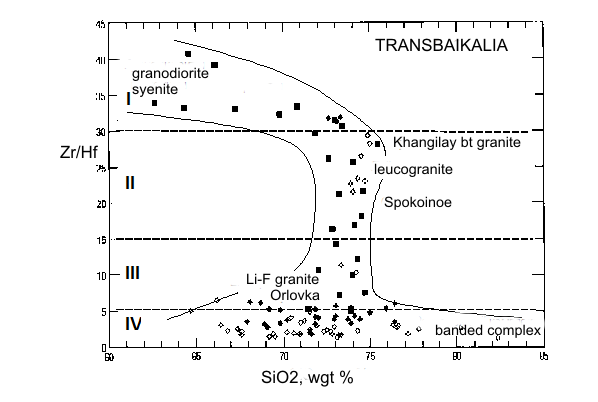
Granites are formed in various geological-tectonic setting at active participation of the water fluid containing other active components: CO2, Cl, F, etc. Fluorine is one of the main parameter of formation of rare metal granite, pegmatite, and related deposits because it influence on melting of rocks and take part in carry and adjournment of many ore elements. The role of fluorine in geological fluids still more not enough quantitative characterized. For quantitative estimations of fluorine concentration in granite fluids, the system experimentally calibrated mineral geofluorimeters has been developed (Aksyuk, 2002). It includes biotite (Bt), muscovite (Ms), Li-mica (Li-mica), apatite (Ap), and topaz (Toz) ones. The geoflurimeters take into account complex structure of natural minerals – solid-solutions and various affinity to fluorine of their principal minals. They allow on structure of a mineral with complex anionic and cationic isomorphism and P-T parameters of mineral formation to calculate values of fluorine concentration in the fluids, being in equilibrium with these minerals. The biotite- and Li-micas geofluorimeters like: log MHF (Bt,Li-mica) =log (XF /(1-XF))Bt-1722/T(K)-1,107*XMg,Li+0,216(Al-2)+0,8958+log aH2O, where Т(К) is temperature on Calvin; МHF is concentration of neutral particle HFo in a fluid, equilibrium with mica, in mol/dm3; XF=F/4, XMg.,Li = (Mg+Li)/Σoct; F, Mg, Li, Al mean numbers of these chemical elements in crystallochemical formula of biotite, calculated on 44 negative electric charges; Σoct is the sum of octahedral sites in the formula aH2O - activity of water in a fluid, taking into account complexity of a natural fluid. Others geofluorimeters have a similar kind: logMHF(Ms)=log(XF/(1-XF))Ms-1722/T(K)-0.272(Li+Mg)+0.216(6-Si)+0,185(Fe+Si-6)+1.419+log aH2O log MHF (Ap) = log (XF /(1-XF))Ap - [3657-5,246 P (kbar)]/T(K) + 0.7 + log aH2O. log MHF (Toz) = log (XF /(1-XF))Toz - 2580/T(K) + 0.85 + log aH2O. Molar concentration, МHF, will be close to total HF concentration in a solution at temperatures above 500оС and pressure up to 5 kbar. At presence of alkalis in a solution it is necessary to take into account the contribution of their fluorides in total concentration. Molar concentration of the dissolved substance in the diluted solution to which the majority of natural fluids concerns, at increased Р and Т can be accepted, as a first approximation, equal: MHFT,P =mHF * ρsolT, P, where mHF is mole concentration, mol/kg H2O; ρsolT,P is density of a solution, kg/dm3. Density of a solution it is possible to accept for the majority of natural fluids on water (HGK model). The analysis of a mode of fluorine with the help developed geofluorimeters has revealed three various trends in lg MHF-T relation, characteristic for granites to which different types of deposits are connected (Fig. 1). Rather low fluorine concentrations are typical for barren granites (Ural) and ones with which associated Cu-porphyry deposits (Aksug, Shahtoma, Santa Rita). With decrease of temperature, concentration of fluorine in fluids is appreciablly reduced. Rather high HF concentrations (up to 0.1 mol/dm3) are typical for rare metal (W-Mo-Sn) deposits such as Akchatau (East Kazakhstan), Spokoinoe (East Transbaikalia). There are HF concentration in fluids of three main granite phases, pegmatite, and greisens remain almost on one enough high level at decrease of temperature from 800о up to 500оС. And, at last, high HF concentration (up to 2 mol/dm3) are characteristic for the fluids related to Ta-Nb deposits such as Orlovka and Etyka (East Transbaikalia) and advanced there “apogranite”, and also for topaz contained granitoids and ongonites. A level of fluorine concentration in fluids and Zr/Hf ratio in rare metal granite, describing their differentiation, closely correlate with each other, being the additional indicator such as deposits and their efficiency on ore (Fig. 2, Table 1).
Fig. 1. Relation of log MHF in fluid and temperature of mica formations at different type of deposits
Fig. 2. Behavior of the Zr/Hf and log MHF indicators on the some rare metal deposits of Transbaikalia Table 1. Relationship of the F- and Zr/Hf indicators and ores in granites
The work was supported by RFBR grants: 06-05-64980, 08-05-00835 and Sci. Scool-3763.2008.5
|