|
EXPERIMENTAL STUDY OF PHYSICO-CHEMICAL CONDITIONS INFLUENCE ON TANTALITE-COLUMBITE SOLUBILITY IN HYDROTHERMAL FLUIDS Korzhinskaya V.S., Zaraisky G.P. Institute of Experimental Mineralogy RAS, Chernogolovka, Moscow region, Russia vkor@iem.ac.ru, zaraisky@iem.ac.ru
Transfer and deposition of tantalum by hydrothermal solutions to amount, sufficient for formation of its deposits, for a long time was assumed by many geologists. However the possibility of Ta transport by water solutions earlier has never been demonstrated experimentally. In the literature the reliable data on behavior of Ta and Nb in hydrothermal solutions are absent. The decision of this question has demanded carrying out of special experimental study in conditions of temperatures, pressure and solution composition corresponding to physical and chemical parameters of postmagmatic processes in domes of Li-F granites stocks. We carry out experiments on solubility of natural columbite-tantalite in fluoride (HF, KF, NaF), carbonate (NaHCO3, Na2CO3) and chlorhydric acid (HCl) solutions with concentration ones from 10-2 up to 2m at temperatures 300, 400, 500оС, pressure 500 and 1000 bar, at the presence of oxygen buffers Ni-NiO and Co-CoO. Monocrystal of the mineral has been selected from quartz–amazonite-mica pegmatoid of the Etyka Ta deposit. Its structure is estimated by microprobe CamScan in wt %: Nb2O5 58.99; Ta2O5 17.70; MnO 13.51; FeO 4.42; TiO2 2.59; SnO2 1.54 ; WO3 1.24; (average data from seven analyses). Experiments by duration of 15-20 days carried out in the platinum ampoules on hydrothermal high pressure apparatus of Tuttle type. Total solubility of columbite-tantalite was determined on loss of weight of the monocrystal by weighing on electronic balances with accuracy ±0.01 mg. A quenched solution was analyzed by ICP/MS and ICP/AES method for Ta, Nb, Mn, Fe, and impurity elements: Ti, W, Sn, K, Na.
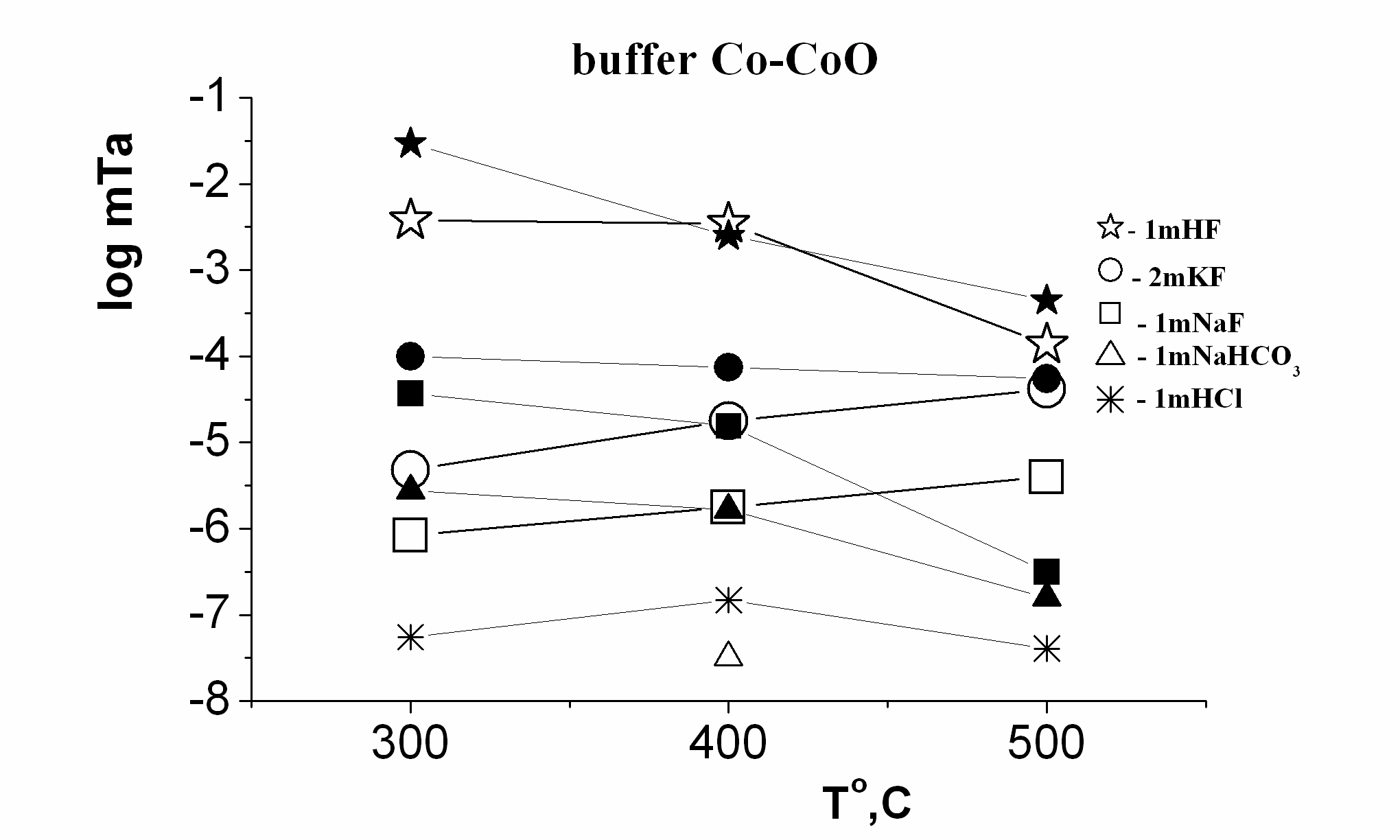
Fig. 1. Temperature dependence of Ta concentration in HF, KF, NaF, NaHCO3, HCl solutions, and Co-CoO buffer at Р=500 bars: dark points, Р=1000 bars: light points.
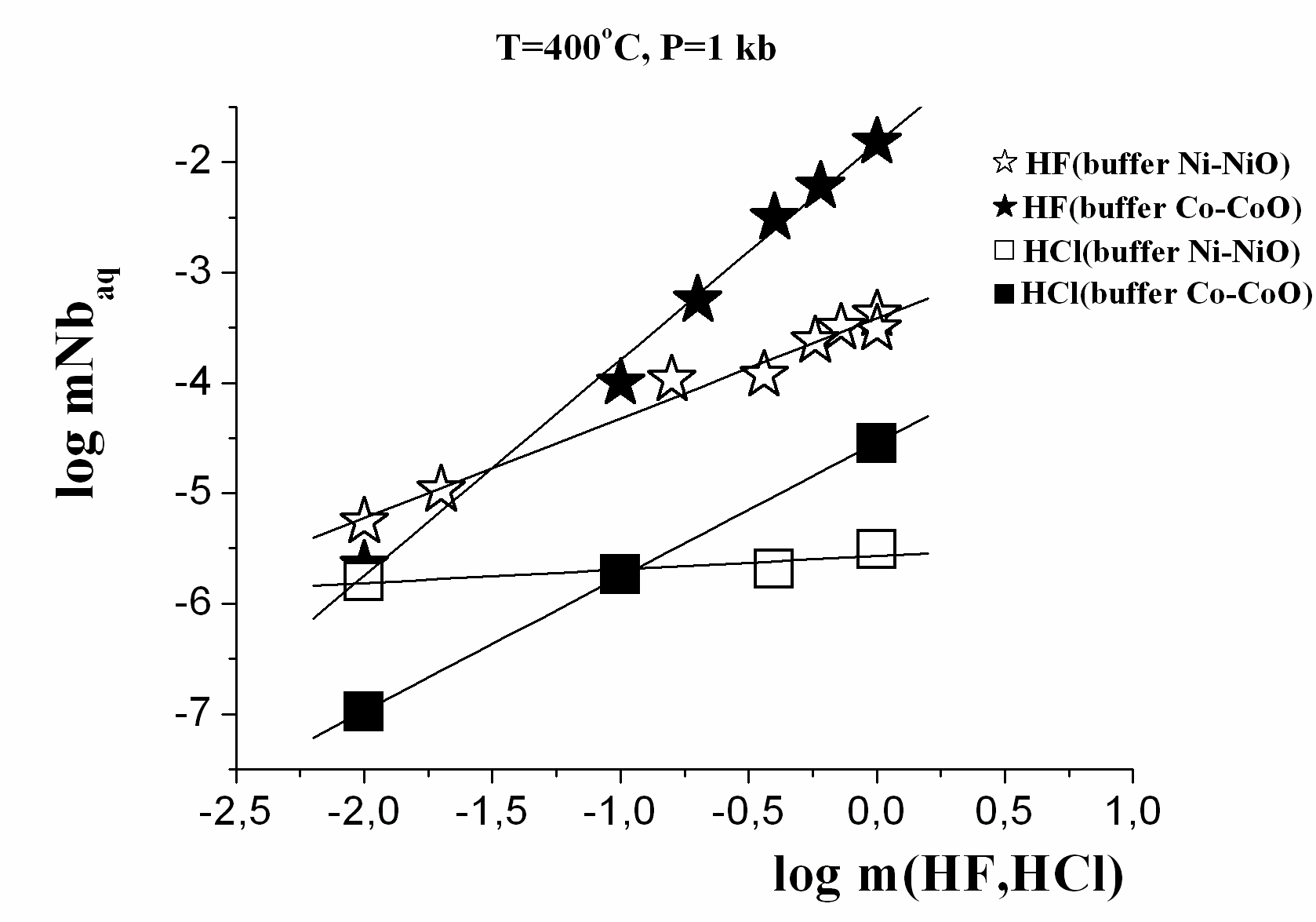
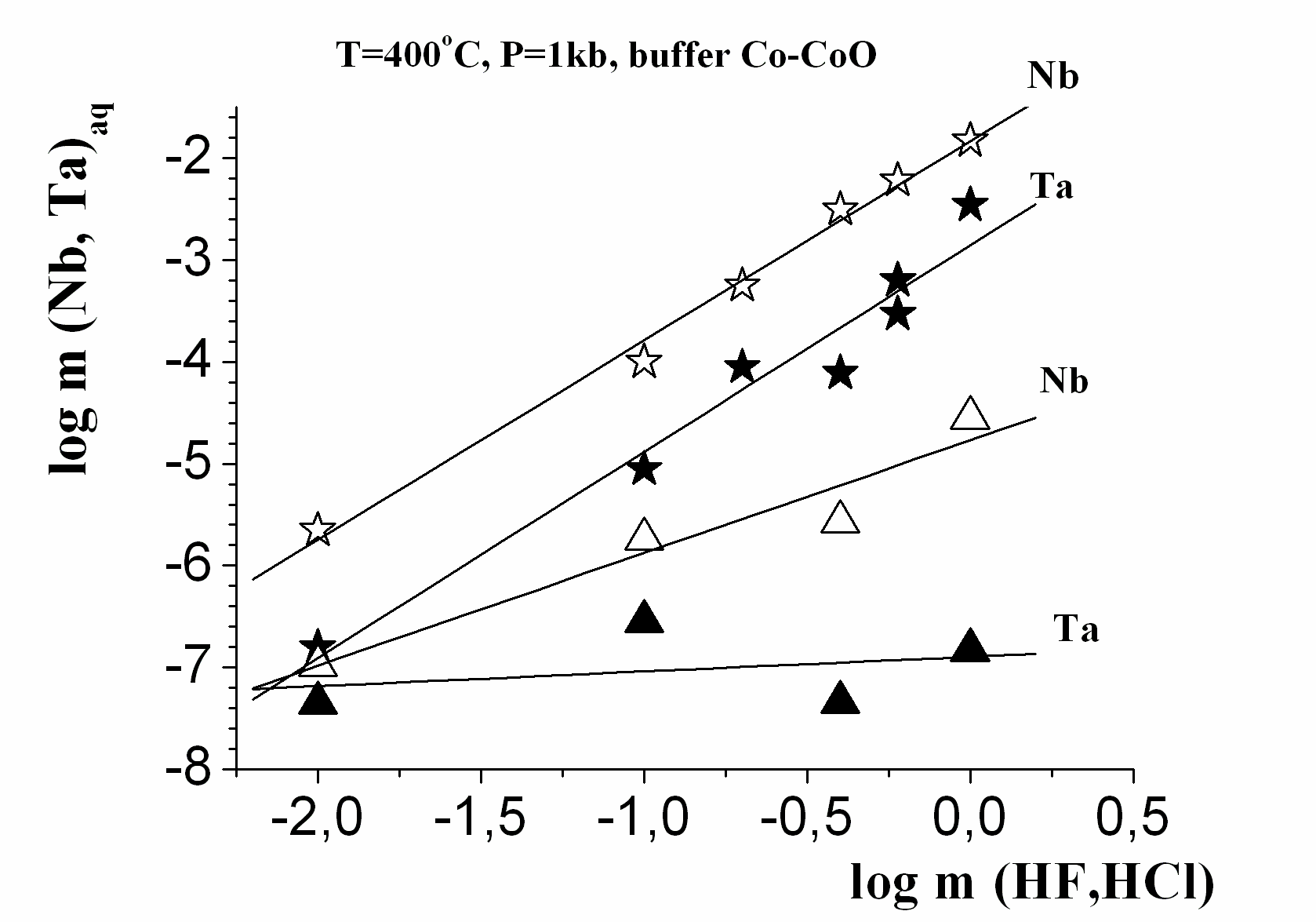
Results of experiments are presented on Fig. 1, 2, and 3. Temperature dependence of the equilibrium tantalum contents is resulted on Fig. 1 for the studied solutions at pressures 500 bar and 1000 bar at the presence of the Со-CoO buffer. It is revealed, that columbite-tantalite has clearly expressed negative temperature dependence, both for Nb and Ta, under all investigated conditions in HF, NaF, NaHCO3 solutions, has weekly expressed in HCl and positive one in KF solutions. Concentrations of Ta and Nb have the highest values up to 10-3-10-2 mole/kg H2O in solutions HF at reduced conditions (Fig. 1). The Nb concentration in the1.0m NaF solution is at 2.5 orders, and Ta one is 3 orders lower than in the 1.0 m HF solution (Korzhinskaya, 2003). The solubility of Ta and Nb in KF solutions has intermediate values. It is established, that in solutions NaF and KF the dependence of solubility on pressure is negative. Strong negative effect of oxygen fugacity increasing on columbite-tantalite solubility was determined. At buffer Ni-NiO, the Ta and Nb concentrations are 1.5-2 orders below than at buffer Co-CoO (Fig. 3). The experimentally established fact of very small solubility of columbite-tantalite in the HCl, Na2CO3 and NaHCO3 solutions is essentially important for understanding of genesis of hydrothermal Ta deposits. In HCl solutions in all a range of the experimentally investigated conditions, the Ta concentration lays in the range from 10-7 up to 10-4 mole/kg H2O (Fig. 2 and 3). In carbonate and bicarbonate sodium solutions the columbite-tantalite solubility is insignificant. Even in 1m Na2CO3 and NaHCO3 solutions it is on the level of 10-5 mole/kg H2O for Nb and 10-8.one for Ta at parameters of the experiments.
Thus, it is possible to speak about real transport of Ta and Nb only by highly enough concentrated fluoride solutions, mainly, HF and, probably, KF ones. Chloride and carbonate hydrothermal solutions are not capable to transfer these metals in the amounts sufficient for formation of mining contents. It explains exclusive date of Ta and Nb deposits in granite and granitic pegmatite only to Li-F type of granite rocks. Financial support by RFBR, projects 08-05-00835 and Science School grant SS– 3763.2008.05 Reference Korzhinskaya V.S., Zaraisky G.P. Solubility of natural tantalite-columbite in fluorine solutions at T = 300 -500oC and P = 1000 bars // Electonic Scienc. Inform. J.”Herald of the Departament of Earth Sciences RAS”, N1(21)`, 2003.
|