|
EXPERIMENTAL STUDY OF Ta2O5 SOLUBILITY UNDER HYDROTHERMAL CONDITIONS RELATED TO THE PROBLEM OF TANTALUM DEPOSITS IN GRANITES Institute of Experimental Mineralogy RAS, Chernogolovka, Moscow region, Russia kotova@iem.ac.ru, zaraisky@iem.ac.ru
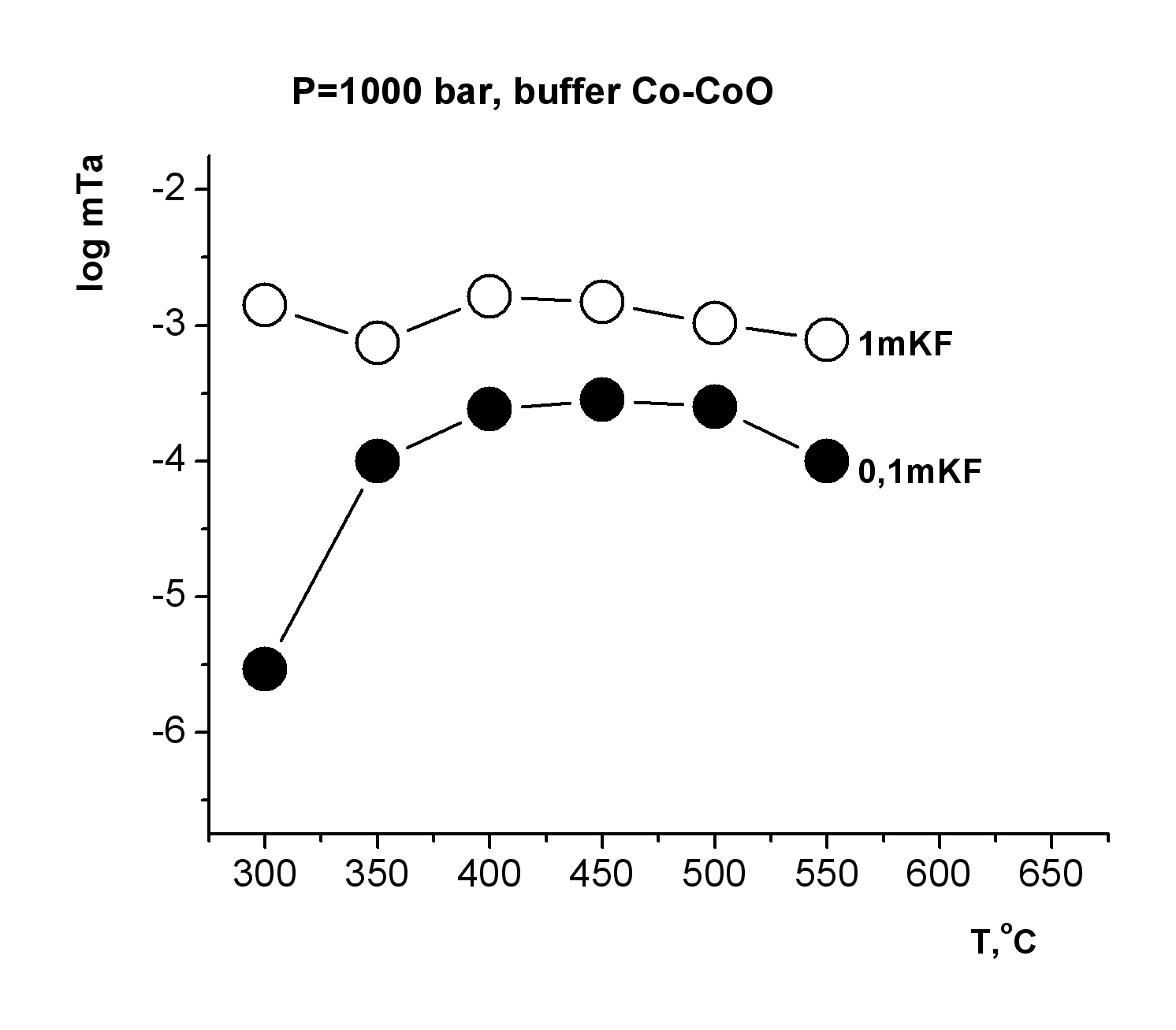
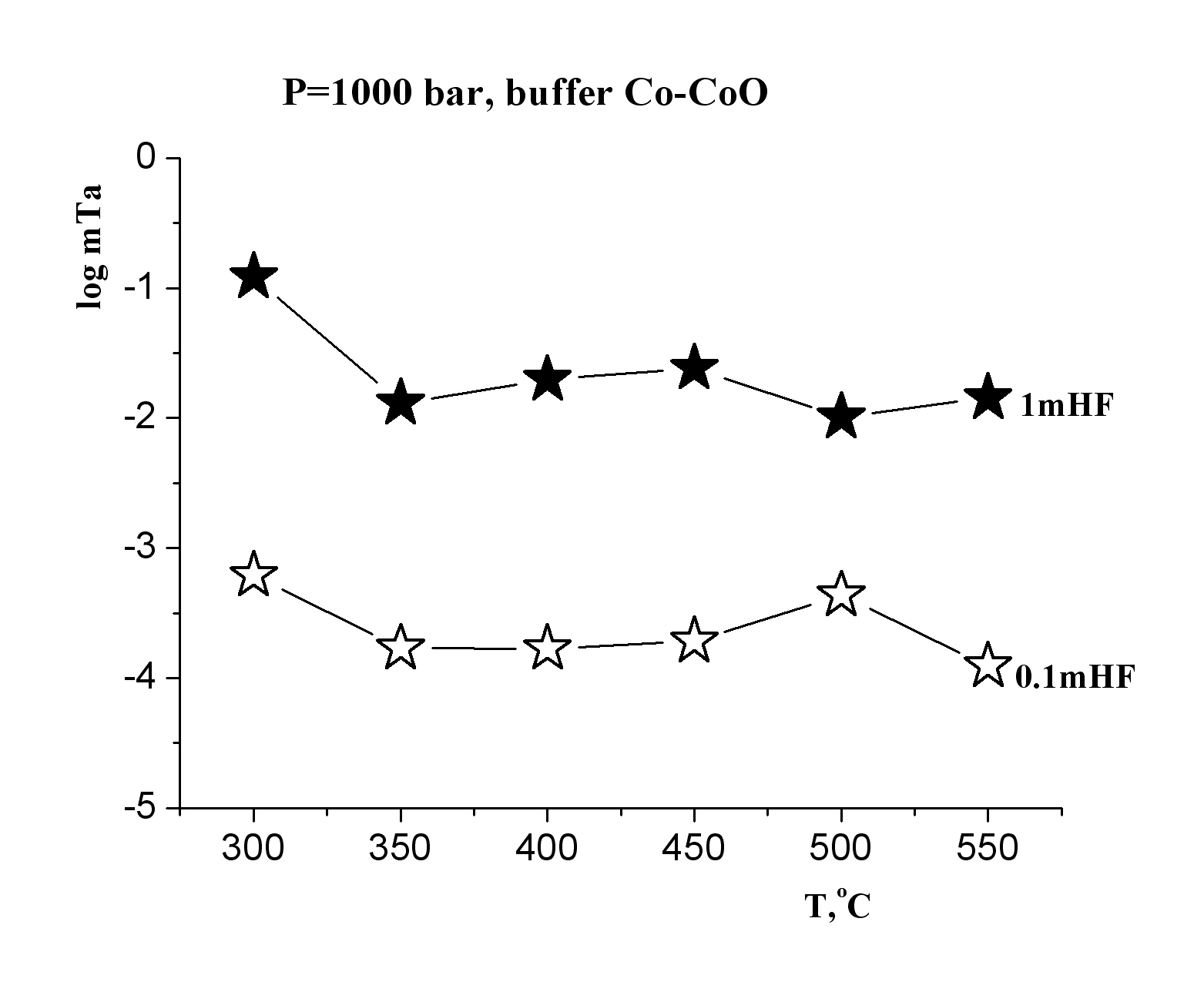
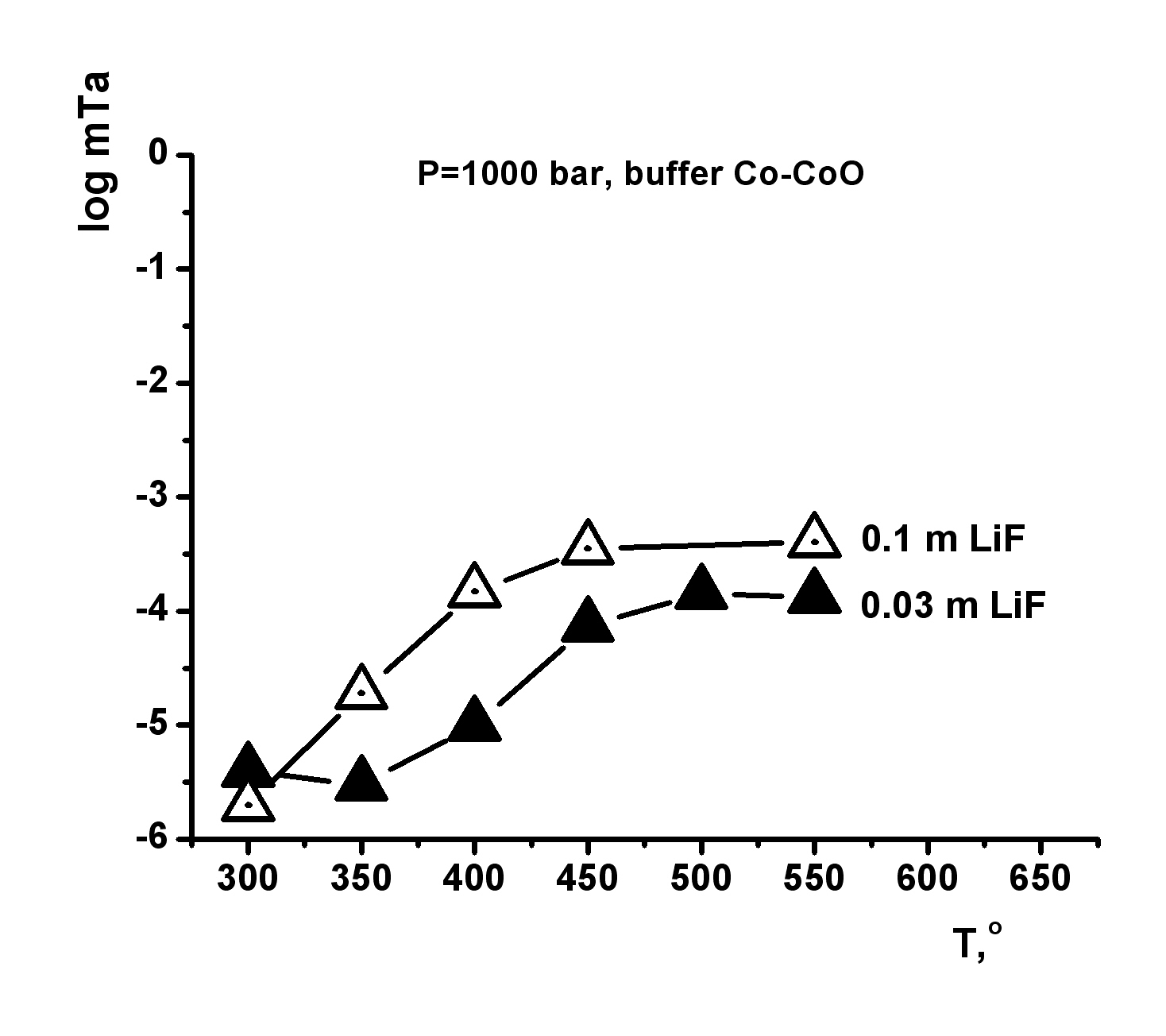
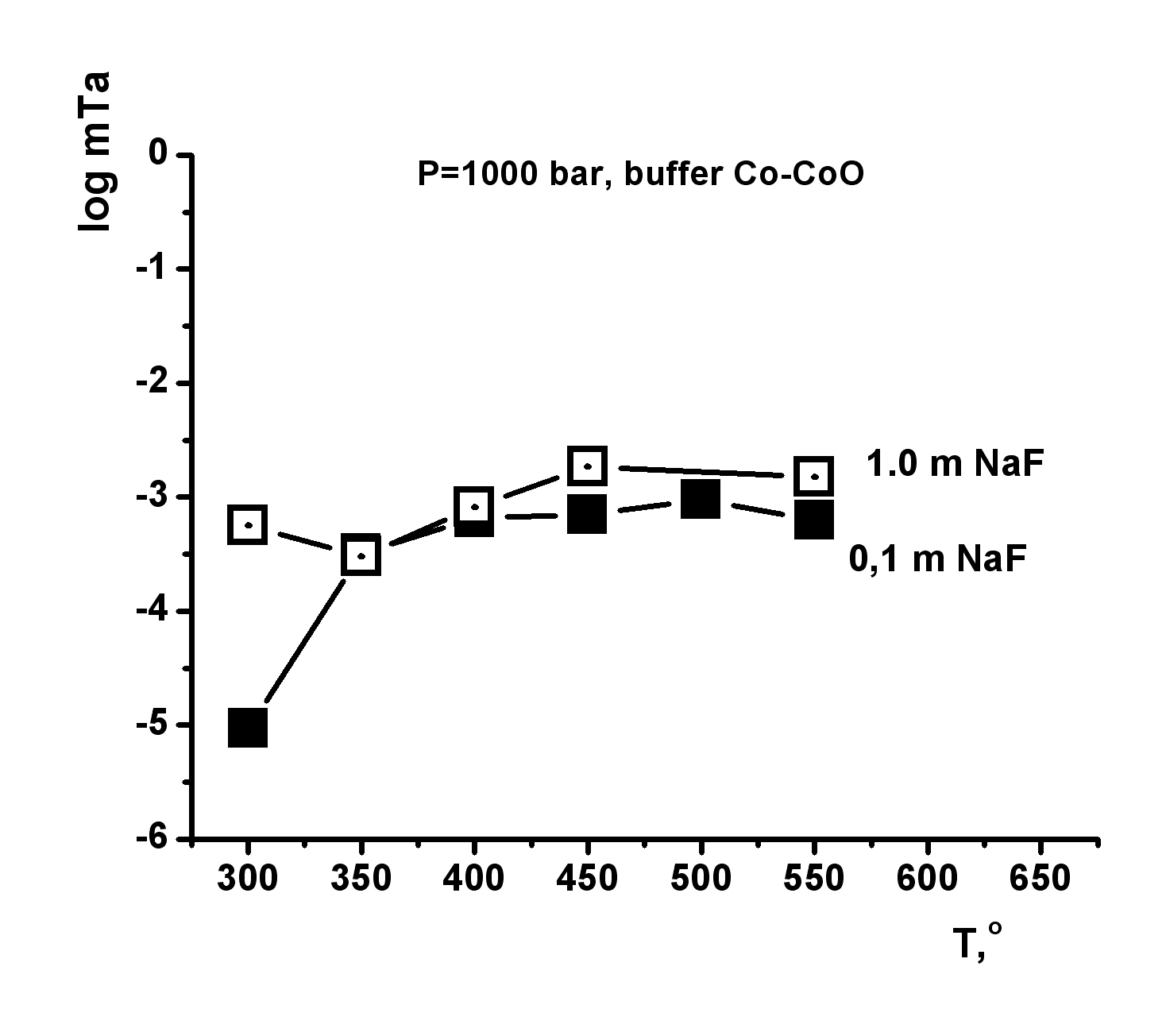
This investigation is directed to solving a specific task of determining the oxide tantalum (Ta2O5-tantite) solubility in fluoride solutions at high temperatures and pressures for quantitative estimation of the possible hydrothermal mass transfer and precipitation of tantalum during formation of its deposits that are associated with albitized and greisenized granite (apogranite) (Beus, 1962). The experiments on studying the dependence of pentoxide tantalum (Ta2O5) solubility on F-ion concentration have been carried out in HF, KF solutions of 0.01-2.0 mole/kg H2Î, NaF solutions of 0.01-1.0 mole/kg H2Î and LiF solutions of 0.01-0.3 mole/kg H2Î at Ò=550oÑ, P=1000 bar (buffer Co–ÑîÎ) (Zaraisky, 2005). Experiments were carried out by a capsule technique in a hydrothermal high pressure apparatus. The run duration was 15 days. The quenched solutions were analyzed for tantalum and impurities by ICP/MS and ICP/AES methods. It has been established that the Ta2O5 solubility isotherms have a similar behavior in all fluoride solutions (HF, NaF, KF, LiF) and have strongly positive trend at low concentrations of fluorides (0.01 and 0.1 mole/kg Í2Î). Tantalum solubility is not very high (10-6-10-5 mole/kg H2O). However, Ta2O5 solubility strongly increases with the increasing F-ion content. It provides the possibility for mass transfer of Ta by hydrothermal solutions. The highest tantalum content was detected in HF solutions (up to 10-1 mole/kg Í2Î) at high concentrations of fluorides (1.0-2.0 mole/kg Í2Î). The dependence of Ta2O5 solubility on HF concentration is close to linear. Ta2O5 solubility was slightly lower in KF solutions (up to 10-2 mole/kg Í2Î in 2.0m KF). Ta2O5 solubility is also high enough in NaF and LiF solutions. The equilibrium concentration of tantalum is within the limits of 10-2 mole/kg H2O in 0.5m NaF and 0.5∙10-3 mole/kg H2O in 0.1m LiF. Temperature dependence of pentoxide tantalum solubility has been studied in HF, KF, NaF solutions with concentration 0.1; 1.0 mol/kg H2O and in LiF solutions with concentration 0.03; 0.1 mole/kg H2O at Ò=300, 350, 400, 450, 500, 550oÑ, Ð=1000 bar and low oxygen fugacity (CO–CoO buffer). The run duration was 30 days at Ò=300, 350o Ñ, 21 days at Ò=400, 450, 500oÑ, 15 days at Ò=550oÑ.
The obtained experimental data have shown that in HF solutions oxide tantalum has congruent solubility throughout the investigated range of temperatures, and its solubility is practically independent on T. The equilibrium concentration of tantalum is within the limits of 10-2 mole/kg H2O in 1.0m HF and 10-4 mole/kg H2O in 0.1m HF (Fig. 1). Temperature dependence of Ta2O5 solubility in KF solutions is also slightly expressed, as well as in HF solutions (Fig. 2). However, in KF solutions, oxide tantalum has incongruent solubility. After the runs the solid products were examined by X-ray diffraction procedures and microprobe investigation. It has been shown that fluorides with formula KxTaO2+xF1-x, where õ is within the limits from 0.4 up to 0.6 are formed. The equilibrium concentration of tantalum is approximately 10-3 mole/kg H2O in 1.0m KF solutions. It is by the order of magnitude lower than that determined in 1.0m HF solutions In NaF solutions the equilibrium concentration of tantalum does not practically change with the increasing temperature, and it is within the limits of 10-3 mole/kg H2O in the whole investigated range of temperatures (Fig. 3). In NaF solutions, oxide tantalum has incongruent solubility, as well as in the case of KF. Na-tantite (Na2Ta4O11) and fluoride of Na-tantalite (Na2Ta2O5F2) are formed. In LiF solutions at T= 300-450oÑ Ta2O5 solubility strongly increases with the increasing temperature (Fig. 4). A temperature increase from 300 to 450oC leads to an increase in tantalum content up to 10-4 mole/kg H2O in 0.03m LiF and 10-4,5 mole/kg H2O in 0.1m LiF. Then it practically stops to change with temperature increase. In the whole investigated range of temperatures, Ta2O5 is congruently dissolved.
The obtained experimental data have shown that in 1.0m fluoride solutions in the most probable a temperature range 400-550oÑ for hydrothermal mass transfer and precipitation of tantalum, the concentration of Ta reaches significant values (10-4- 10-2 m.). It is evidence of the solubility of Ta2O5 depends on F-ion content and also demonstrates the importance of the complexing agents, especially fluorine, in the transport of such elements as tantalum. Financial support by RFBR, projects 08-05-00835-a and Science school grant SS-3763.2008.05
References Beus A.A., Severov E.A., Sitnin A.A. and Subbotin K.D., 1962, Albitized and greisenized granites (apogranites): Moskva, Akademiia Nauk SSSR, 196p. (in Russian). Zaraisky George P., Korzhinskaya Valentina S., Kotova Nataliya P. The problem of hydrothermal transport of tantalum and niobium in “apogranites” on experimental data – Understanding the genesis of ore deposits: To meet the demands of the 21st century, 12th quadrennial IAGOD Symposium, 2006, Moscow, P.21-24 August, 2006.
|